TechnologyOur technology
Crystal Structure Control Technology
The products developed and manufactured by Tanaka Chemical Corporation include nickel hydroxide and, generally, all metallic compounds. Even if these metallic compounds are the same (compounds with the same chemical formula), their properties can differ greatly due to differences in crystal structure. We have succeeded in changing the crystal structure of our products and producing materials with crystal structures tailored to various battery applications.

| Overview | The terms "high" or "low" crystallinity are used to describe how regular the crystal structure is. In the case of battery materials, the optimal crystallinity differs depending on the type of battery and the design of the manufacturer. For example, precursors for lithium-ion batteries often require highly crystalline materials (in a lattice structure with regularly arranged atoms), while low crystallinity cathode materials (in an irregular structure, lacking uniformity and consistency) for nickel-metal hydride batteries are, to some extent, more suitable. While developing cathode materials and precursors, we also control the crystallinity, by implementing reaction control based on our proprietary expertise. |
|---|---|
| Advantages | Contributes to improved battery output by controlling the ease of chemical reactions in the battery during charging and discharging |
| Utilized in | Lithium-ion (Li-ion) battery Nickel-metal hydride (Ni-MH) battery |
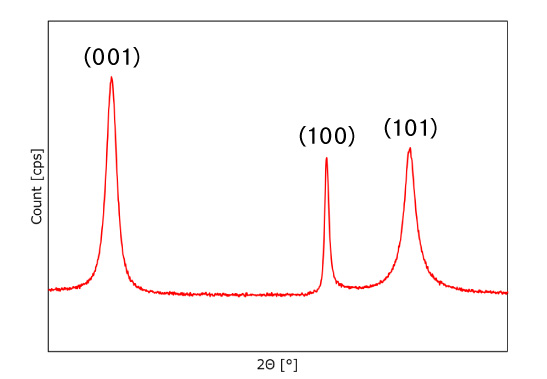
Fig.1 NiCoMn precursor X-ray diffraction pattern
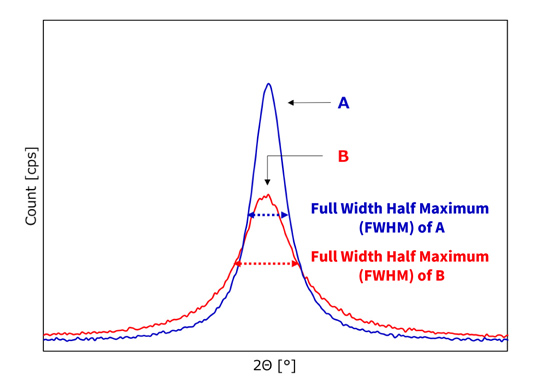
Fig.2 Comparison of NiCoMn precursors with different crystallinity
Figure 1 shows the powder X-ray diffraction pattern of Co and Mn coprecipitated nickel hydroxide, with no impurity peaks other than those characteristic of β-type nickel hydroxide, indicating that Co and Mn are doped at the atomic level to the matrix structure of nickel hydroxide. Figure 2 shows comparative data of two of our products with the same composition but with different crystallinity. Since the products have the same composition, the position of the peak is the same, but the Full Width Half Maximum (the spread of the peak at 1/2 height: indicated by the dotted arrows) is different between A and B. If the crystals are defect-free and arranged in a periodic pattern, the X-ray beam is diffracted at the same angle depending on the crystal planes of the sample, but if the periodicity of the crystals is low, the peak is broader and the Full Width Half Maximum (FWHM) is wider. This means that A is highly crystalline while B is less crystalline.
